Buttons
Info
USP <800>
Like USP General Chapter < 797> on Pharmaceutical Compounding – Sterile Preparation, USP General Chapter <800> provides for and requires all the same standards found in <797> but adds a long list of additional requirements for the safe handling of hazardous drugs to minimize the risk of exposure to healthcare personnel, patients and the environment.
The National Institute for Occupational Safety and Health (NIOSH) considers a drug to be hazardous if it exhibits one or more of the following characteristics in humans or animals: carcinogenicity, teratogenicity or developmental toxicity, reproductive toxicity, organ toxicity at low doses, genotoxicity, or structure and toxicity profiles of new drugs that mimic existing hazardous drugs.
USP General Chapter <800> describes requirements including responsibilities of personnel handling hazardous drugs; facility and engineering controls; procedures for deactivating, decontaminating and cleaning; spill control; and documentation. These standards apply to all healthcare personnel who receive, prepare, administer, transport or otherwise come in contact with hazardous drugs and all the environments in which they are handled.
Like our complete line of Pharma-Brand products covering your USP <797> requirements, Acute Care Pharmaceuticals has been working hard to complete our line of environmental and personal protection products to satisfy the new requirements for USP <800>. Call us today to receive our full line catalog of HD safety products.
Featured Products
FEATURED PRODUCTS
USP General Chapter <800> Hazardous Drugs - Handling in Healthcare Settings
USP provides standards for safe handling of hazardous drugs to minimize the risk of exposure to healthcare personnel, patients and the environment.
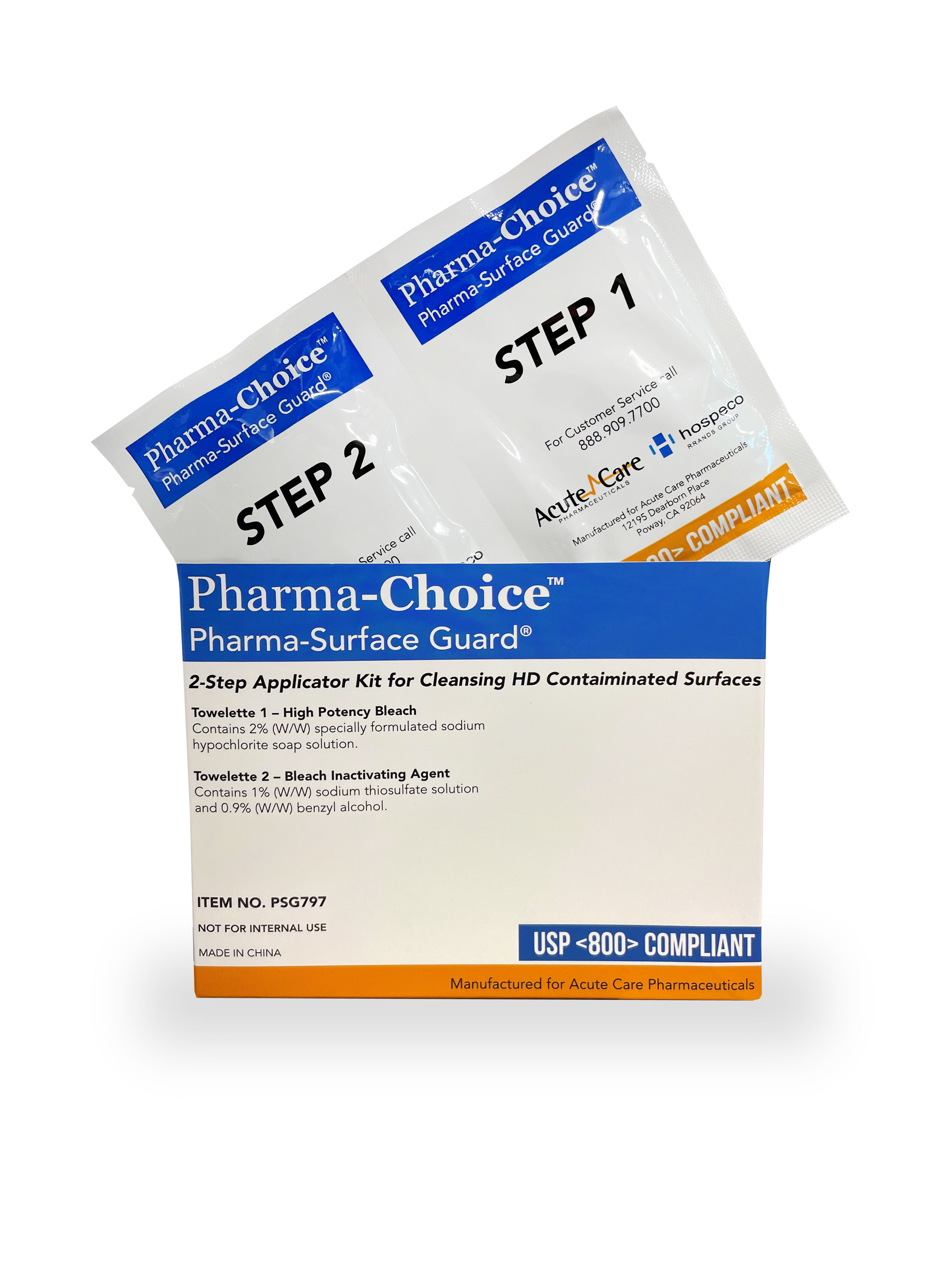
Pharma-Surface Guard®
Cleans and Deactivates Most Chemotherapy Drugs and other HDs
USP <800> - PRODUCTS

Gloves / PPE

Cleanroom / Critical Environments